|
Definition: "An ergogenic aid is any substance or phenomenon that enhances performance "
|
|
||||||||
26.09.2011 |
|
|
Why raloxifene is safer than tamoxifen
When used over long periods, tamoxifen, the active ingredient in the anti-oestrogen Nolvadex, can increase the risk of estradiol-related cancer. This is because it boosts the conversion of estradiol into potentially carcinogenic metabolites, write researchers from the University of Texas in the Journal of Steroid Biochemistry and Molecular Biology. It's a near relative raloxifene – the active ingredient in Eli Lilly's Evista – actually lowers the risk of cancer, according to the American study.
Doctors subscribe tamoxifen for women who are breast cancer survivors. Long-term anti-oestrogen treatment dramatically reduces the likelihood of the cancer returning. But a few years ago doctors discovered that tamoxifen leads to an increased risk of endometrial cancer. [Gynecol Oncol. 2004 Aug; 94(2): 256-66.] [J Natl Cancer Inst. 2005 Mar 2;97(5):375-84.] Raloxifene, an anti-oestrogen that works in almost the same way as tamoxifen, actually reduces the risk of endometrial cancer. [J Clin Oncol. 2008 Sep 1; 26(25): 4151-9.]
Tamoxifen works in two ways. The first is that the molecule attaches itself to the estradiol receptor and then sabotages the receptor. The second is that tamoxifen stimulates the production of enzymes that convert estradiol into less active analogues. And it's in this latter mechanism where the problems lie. Through this tamoxifen boosts the concentration of 4-hydroxy and 16-hydroxy analogues of estradiol. They can attach themselves to the DNA – forming DNA adducts – and in this way damage the genetic material. If a cell cannot repair the damage, it can change into a cancer cell.
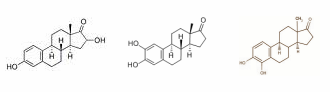
Below from left to right you see 16-hydroxy-estrone [risky], 2-hydroxy-estrone [not risky] en 4-hydroxy-estrone [risky].
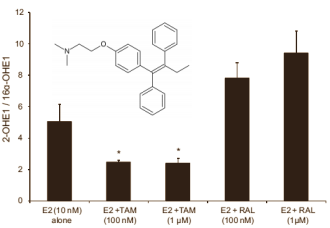
The Americans confirmed this theory in their in-vitro study. They exposed human endometrium cells to estradiol [E2], estradiol and tamoxifen [Tam], and estradiol and raloxifene [Ral]. The combination of estradiol and tamoxifen did indeed result in an increased number of risky estradiol metabolites. The figure below, for example, shows that the ratio of 2-hydroxy-estrone (harmless) to 16-hydroxy-estrone (dangerous) decreases.
When the researchers analysed the cells' DNA they noticed that the estradiol-tamoxifen combination [structural formula shown below] had led to an increase in the number of DNA adducts.
The figure above suggests that tamoxifen boosts the synthesis of the enzyme CYP1B1 and reduces the synthesis of the enzyme catechol-O-methyltransferase COMT. CYP1B1 converts estradiol into dangerous steroids; COMT helps cells to deactivate dangerous estradiol metabolites.
The effect is so subtle that short-term use of tamoxifen probably has no great consequences. But for health-conscious chemical athletes, who want to use an anti-oestrogen for longer periods, it could well be a reason to choose something other than tamoxifen.
Source:
More:
|
|